You have undoubtedly come across dextrose in your travels. This sugar, which is also known as glucose, is fundamental to almost all forms of life on the planet.
Also known as brewer's sugar, dextrose is converted quickly and completely to alcohol. Because of this, it leaves no residual sweetness and contributes to the 'dry' character of a fermented beer.
Dextrose is used by the yeast to produce energy. Other sugars, e.g. maltose which is two glucose molecules combined (more on that later), have to be converted to glucose before they can enter the Krebs cycle; this fact is true for humans as well.
Plants and algae also produce dextrose when they photosynthesize, so dextrose is an important molecule for 'life as we know it', not just making beer. Because all life uses dextrose to some extent, it is a fundamental unit of many larger 'complex' carbohydrates.
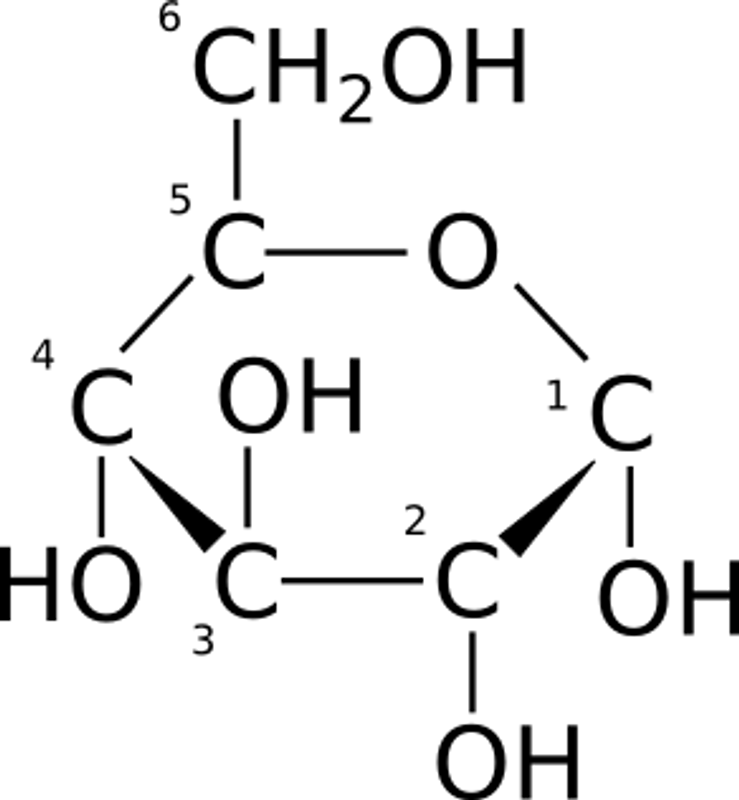
Dextrose is comprised of six carbons, which can exist as a long straight chain or as a number of different shaped ring structures.
It is present in ring form a majority of the time and these rings can consist of five or six carbons in a variety of twisted shapes, but is often illustrated as a tidy 6-membered (pyranose) ring pictured to the upper right.
An interesting thing about glucose (which is true all throughout biology) is that it can have a left or right hand configuration (chirality). D-glucose (or dextrose) is pictured on the left. On the 'left handed' L- glucose, the H and OH attached to carbon number two are reversed.
Here on earth, life has evolved to only use the right-handed form of the molecule. When a pure sample is placed in polarized light, it will rotate the direction of this polarization to the right (dextro-rotation) and this is where the name D-glucose or dextrose is derived.
The left handed (levo-rotation) version of glucose has been synthesized in the lab and even tastes sweet. However, due to the shape of the molecule, it won't fit into the enzymes which perform the Krebs cycle.