Yeast might make the beer, but it needs a brewer to create the wort. In the wort is a mélange of sugars most of which are derived from starch during the mash. A bit of knowledge of the different sugars (carbohydrates) produced during this process helps to explain what happens in the mash and how the yeast consumes these sugars.
This is all about the sweet stuff.
To most people, sugar is the name of the sweet granules we sprinkle on our cornflakes in the morning. To be more specific table sugar is also called sucrose and is one small part of a huge family of organic chemicals known as carbohydrates.
Carbohydrates exist all throughout nature; dextrose or glucose is the molecule where we derive all our energy. Wood and paper are made of cellulose which is a polymer comprised of dextrose molecules. Starch, also a polymer of dextrose molecules is a form of energy storage in plants, and the addition of a carbohydrate to a protein (known as glycosylation) is a process which happens in each of us thousands of times a day.
The starch in a kernel of barley or another brewers adjunct is insoluble and too massive for the yeast to consume as it is, mashing malted grains breaks these grains of starch down in to soluble sugars some of which the yeast can consume.
Mastering the mash.
You might have heard the phrase ‘enzymatic hydrolysis of starch’ thrown around to describe the process occurring in the mash.
Another way of stating this is we are using enzymes present in the malted grains to break down the starch granules to shorter chain sugars, which will dissolve in to the wort to be consumed to the yeast.
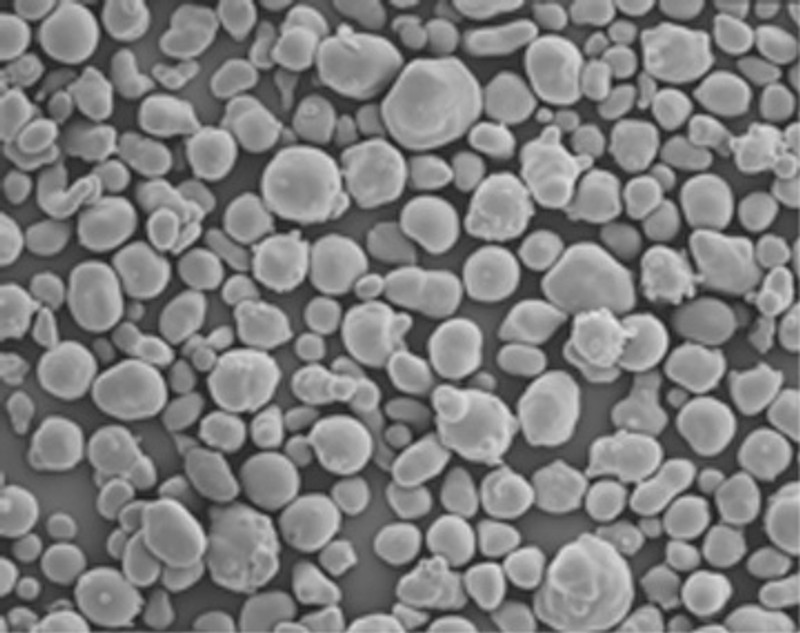
Granules of starch (pictured above) are comprised of two species of carbohydrate; amylose and amylopectin. Both of these molecules are described as polymers, which is to say they are comprised of many repeating units (mers) for these two that mer is dextrose.
Glucose:
You have undoubtedly come across glucose in your travels. Glucose is a sugar molecule containing six
carbons, which can exist as a long straight chain or as a number of different shaped ring structures.
It is present in ring form a majority of the time and these rings can consist of five or six carbons in a variety of twisted shapes.
The six membered form is often illustrated as tidy little rings as to the right.
Aside from the linear or ring form, glucose has two possible configurations depending on which direction the oxygen bound to carbon 1 points –right and left ‘handed’.
Here on earth as life has evolved to only use the right-handed form of the molecule. When a pure sample is placed in polarized light it will rotate the direction of this polarization to the right (dextro-rotation) this is where the name D-glucose or dextrose is derived from.
The left handed (levo-rotation) version of glucose has been synthesized in the lab and even tastes sweet however due to the shape of the molecule it is incompatible with enzymes used to break down dextrose to harness it’s energy and so it has no nutritional value.
Maltose, maltotriose and dextrins:
Maltose is the sugar molecule close to every brewers heart, comprised of two dexstrose molecules whereas maltotriose is made up of three.
These are joined by an ‘α1->4 glycosidic linkage’ which we will go in to more detail about soon. The short story is that carbon 1 in the first ring bonds with carbon 4 in the second.
Glucose, maltose and maltotriose make up the majority of the wort and these are what we refer to as the ‘fermentable fraction’. The ability to consume maltotriose on the right is the hall-mark of a highly attenuating strain of yeast.
These glycosidic linkages, as in glycosylation mentioned above join sugar molecules to something else. They can come in one or two configurations α/β depending on the physical orientation of the molecules, similar to the idea of L and D glucose.
You can use these to tack glucose molecules in to intermediate length chains of three to nine units (oligosaccharides) all the way in to their thousands (polysaccharides). Cellulose is a polysaccharide of glucose molecules linked by a β1->4 configuration, because of the different shape of the glycosidic linkage though it can’t be broken down by amylase.
Chains of 4 – 20(ish) glucose molecules joined in the same way as maltose and maltotriose are known as maltodextrins. These contribute largely to the non-fermentable portion of wort, which is why it is often used for sweetening.
Amylose and amylopectin.
When your chain of α1->4-linked dextrose molecules starts to number in the thousands you can safely say you have an amylose molecule.
α1->6 linked glucose can also be added along the length of the α1->4 linked back-bone, when these occur the polysaccharide is known as amylopectin.
Linked like this it becomes a straight (linear) tightly packed polymer, which winds in upon itself to form a helix.
When malt is described as well modified it means the majority of amylopectin has been converted to amylose by the embryonic plant during the malting process.
These side branches occur around every 24 to 30 units and prevent tight packing of the molecule like in Amylose. These are only broken down by γ-amylase.
Glycogen which is a sugar storage molecule in humans and yeast has the same branching arrangement configuration as amylopectin, only with side chains every 10 glucose units.
Molecules of amylopectin and amylose are bound with protein to form the starch granule pictured above. This loose packing means that amylopectin gels faster than amylose.
In the process of gelling the starch granule swells and bursts which expels molecules of amylose and amylopectin into the mixture. Water associates with the hydroxyl (-OH) groups on the mers, which thickens the mash similar to creating flour paste.
From here the amylase enzymes get to work breaking the glycosidic links in the amylose and amylopectin to produce the small soluble (and hopefully fermentable) sugars above.
Other carbohydrates.
There are a few other sugars, which can find their way in to beer (although in some places there are laws to prevent this).
The first to mention is fructose also called fruit sugar. This is another six carbon sugar (a hexose) which is very similar to glucose.
Fructose unlike glucose however is a non-reducing sugar and doesn’t behave completely the same. It also tends to favor five membered ring structures.
Together glucose and fructose form every bodies friend disaccharide friend sucrose via a β1->2 linkage. This is broken down into its components before it enters the yeast by an enzyme called invertase (which we will take about another time). It is this enzyme though which gives name to ‘invert’ sugar which is simply a mixture of glucose and fructose.
As the ratio of fructose to glucose in sucrose is 1:1 when this is broken down you get even quantities of the two in invert sugar. This ratio is close to that found in honey and some blends of high-fructose corn syrup.
Another hexose we need to introduce is galactose with its ring structure pictured to the left.
Although it can be consumed by yeast, and is a component of structural carbohydrates found in the husk of grain it isn’t particularly important to brewers on its own.
Paired with glucose it forms two disaccharides of interest to brewers; lactose and melibiose.
Lactose (pictured right) the sugar found in milk, is used as a sweetener for milk-stouts. Yeast does not possess the lactase enzyme required to break the glycosidic link and so this sugar is non-fermentable. It is a disaccharide of galactose and glucose with a β1->4 linkage.
Melibiose (pictured left) in the other hand is glucose and galactose joined by an α1->6 linkage.
‘True’ lager yeasts possess an enzyme known as melibiase which breaks the glycosidic linkage which allows the yeast to consume the components, it is this process which occurs during the low temperature lagering period which contributes to the crisp, dry body of many lagers.
Why we do while we brew.
Obviously you don’t need this kind of technical information for putting down a brew, formulating recipes or drinking beer!
Knowing that glucose is the building block for many of these sugars and understanding that this can form long chains is important for describing what you are actually doing while you are mashing the grain.
In part II of this article we will discuss how enzymes operate and mention some of the important ones used during mashing, and conclude with the important information on how the yeast actually consumes these sugars.